What is ABvac40?
ABvac40 is a vaccine developed to prevent Alzheimer’s disease from its earliest stages. Phase 2 clinical studies have shown promising results, indicating that the vaccine is safe and capable of generating a strong and sustained immune response over time. In addition, some patients have experienced some cognitive benefits, although these findings need to be further evaluated in subsequent clinical trials.
Vaccine development and origin
The idea of creating a vaccine to prevent Alzheimer’s was conceived by Professor Manuel Sarasa, professor at the University of Zaragoza (Spain) and founder of Araclon Biotech. After years of preclinical research in the laboratory, the first clinical phase began in 2015 with 24 participants, focusing mainly on the study of safety and how the immune system responded to the treatment. Thanks to the good results obtained, the Spanish Medicines Agency (Agencia Española del Medicamento) authorised to advance to phase 2 of the clinical trial (the AB1601 study).
How does ABvac40 work?
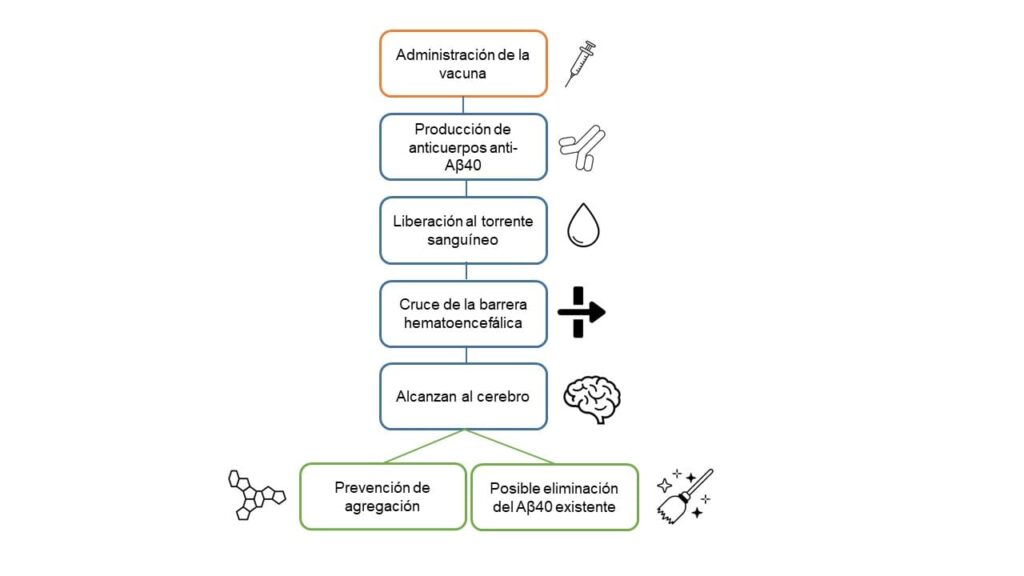
ABvac40 is an active vaccine designed to generate antibodies against amyloid beta peptide 40 (Aβ40). This peptide (Aβ40 or beta-amyloid 40) is a fragment of a larger protein called amyloid precursor protein (APP). Under normal conditions, the body produces these proteins, but in people with diseases such as Alzheimer’s and Cerebral Amyloid Angiopathy (CAA), Aβ40, along with its variant Aβ42, tends to accumulate abnormally in the brain.
In Alzheimer’s disease, these peptides aggregate to form amyloid plaques or deposits, a hallmark of the disease that contributes to neuronal dysfunction, inflammation and, eventually, the death of neurons, resulting in the cognitive impairment typical of the disease.
In the case of AAC, Aβ40 is mainly deposited on the walls of blood vessels in the brain, which can cause their weakening and increase the risk of brain haemorrhages.
By stimulating the body to produce specific antibodies against this peptide, the vaccine could help prevent or reduce the build-up of Aβ40 in the brain, which could slow the progression of both diseases.
Unlike other therapies available outside the European Union, ABvac40 has so far proven to be a very safe treatment, with no serious adverse effects associated with treatment in the study phases conducted.
Latest results from ABvac40
The AB1601 study, a phase 2 clinical trial involving 124 patients with amnestic mild cognitive impairment or very mild Alzheimer’s disease, was recently completed. This study was conducted in several European countries, including Spain, France, Italy and Sweden. The results obtained in this study confirm previous findings on the safety and tolerability of ABvac40 in early Alzheimer’s disease, and confirm that it induces a strong immune response.
During the AB1601 trial, patients treated with ABvac40 showed slower cognitive decline than those treated with placebo, as measured by their scores on different cognitive tests. These tests are a series of assessments designed to measure people’s cognitive abilities, and are carried out by trained medical professionals.
Among the most common is the Mini-Mental State Examination (MMSE), which showed better scores in vaccine-treated patients than in placebo-treated patients, suggesting a possible benefit in cognitive function. In addition to the MMSE, other neuropsychological tests, such as the Trail Making Test (TMT-A), were used to assess patients’ attention and mental processing speed. In these tests, some participants also showed positive results, suggesting that the ABvac40 vaccine could help slow cognitive decline in people with early-stage Alzheimer’s disease. However, these results are exploratory, and more studies with larger numbers of patients will be needed to confirm the vaccine’s efficacy in these areas.
When could an Alzheimer’s vaccine be available?
Although more clinical trials are needed, we are confident that, if all progresses favourably, ABvac40 could be available within the next decade.


